Biogen Completes Submission of Biologics License Application to FDA for Aducanumab as a Treatment for Alzheimer's Disease
- Written by ACN Newswire - Press Releases

Read more //?#

Read more //?#

financial accountability to how they run cloud and AI, according to leading Australian systems integrator, Brennan. Based on customer insights...

UNSW Launches Global Innovation Foundry to Scale 100 Australian Startups Internationally New initiative provides startups and spinouts with direc...
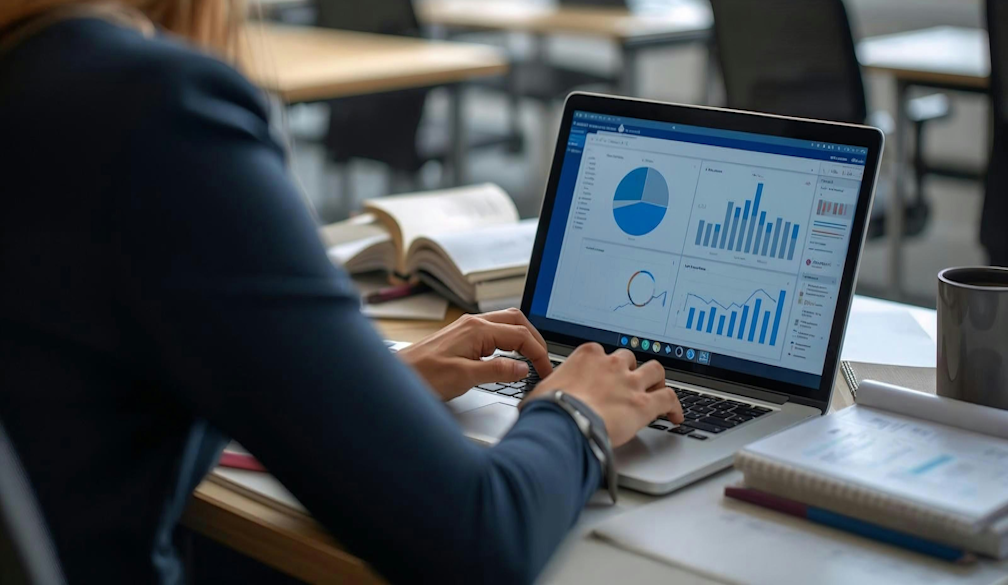
A year after wage theft reforms came into effect, Australian businesses have increased their focus on payroll compliance, but confidence in pay accu...

Australian retailers are turning to artificial intelligence to simplify and automate returns and exchanges, while strengthening loyalty programs a...

I’ve been in customer service for years now. As my team has grown, the number one piece of advice I give is to be your...

Richard Valente, VP of Customer Experience Strategy at TP in Australia, explores how IT-BPM outsourcing is revolutionising the travel sector throu...